![[ The Methionine Cycle ]](MethCyc1.jpg)
![[ The Methionine Cycle ]](MethCyc2.jpg)
by Ben Best
A picture is worth a thousand words, as the saying goes, but one picture does not necessarily tell the whole story. For the methionine cycle, I think it is best to give an introduction in words followed by several diagrams which show the cycle in different ways, and with different branches.
Methionine is a sulfur-containing amino acid which enters the body through dietary proteins, is used in forming proteins in the body, and is the precursor (through the methionine cycle) of the sulfur-containing amino acids homocysteine, cysteine, and taurine. Methionine has a methyl (−CH3) group attached to its sulfur atom. The methyl group on methionine is used for adding methyl groups to numerous kinds of molecules, but only after methionine has been activated.
In the Methionine Cycle, methionine is converted to SAMe (S−Adenosyl Methionine), which is a methyl donor for numerous reactions. In losing its methyl group, SAMe becomes SAH (S−Adenosyl Homocysteine), which is then converted to homocysteine. Homocysteine is either converted back to methionine, or it enters the transsulfuration pathway to form other sulfur-containing amino acids.
In more detail: in the Methionine Cycle, methionine's methyl group becomes activated by ATP (adenosine triphosphate) with the addition of adenosine to the sulfur of methionine, adjacent to the methyl group to form S−Adenosyl Methionine (SAMe). Removal of the methyl group from SAMe results in the formation of S−Adenosyl Homocysteine (SAH), which is immediately converted to the amino acid homocysteine by removal of the adenosine molecule. Homocysteine can then be modified in three different ways. In liver cells (and only in liver cells) homocysteine can irreversibly enter the transsulfuration pathway (catalyzed by Vitamin B6) to produce the amino acid cysteine. It has been estimated that 60% of homocysteine is metabolized by transsulfuration in the liver, with glucocorticoids increasing that percentage [HUMAN MOLECULAR GENETICS; Ulrey,CL; 14:R139-R147 (2005)]. Cysteine can be incorporated into proteins, can be used in the formation of the anti-oxidant molecule glutathione (GSH), or can be oxidized to form the amino acid taurine. Ames dwarf mice (which have extended lifespans) show enhanced activity of the transsulfuration pathway, and significantly increased liver glutathione [MECHANISMS OF AGEING AND DEVELOPMENT; Uthus,Eo; 127(5):444-450 (2006)]. If homocysteine does not enter the transsulfuration pathway, it can be converted back to methionine by the addition of a methyl group by one of two pathways. For one pathway of methyl group addition, methionine synthase enzyme catalyzes the transfer of a methyl group from methylated folic acid (MethylTetraHydroFolate, MTHF) to homocysteine assisted by vitamin B12, which takes the methyl group from MTHF and adds it to the homocysteine. In another pathway (only in the liver), betaine (TMG) is the source of the methyl group transferred to homocysteine. Selenium deficiency increases transsulfuration of homocysteine, and decreases global DNA methylation [JOURNAL OF NUTRITION; Davis,CD; 133(9):2907-2914 (2003)].
![[ The Methionine Cycle ]](MethCyc1.jpg)
|
![[ The Methionine Cycle ]](MethCyc2.jpg)
|
![[ The Methionine Cycle ]](MethCyc3.jpg)
|
![[The Methionine Cycle ]](MethCyc4.jpg)
|
![[The Methionine Cycle ]](MethCyc5.jpg)
|
![[The Methionine Cycle ]](MethCyc6.jpg)
|
|
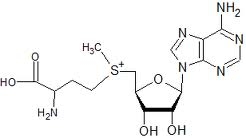
SAMe (pronounced "Sammy") is a molecule composed of adenosine (derived from ATP) and methionine. SAMe is the primary methyl (−CH3) donor in metabolism, which donates a methyl group (methylation) to molecules such as DNA, proteins, phospholipids, or neurotransmitters. DNA methylation is the means of epigenetic control of gene expression. Donation of the methyl group transforms SAMe into SAH (S−Adenyl Homocysteine), which is a potent inhibitor of methylation. For this reason, the SAMe/SAH ratio can be used as an index of methylation potential in a cell [BIOFACTORS; Williams,KT; 36(1):19-24 (2010)]. Ethanol inhibits methionine synthase, depleting GSH in the brain & liver, and decreasing the SAMe/SAH ratio [NUTRITION REVIEWS; Schalinske,KL; 63(11):387-391 (2005) and ALCOHOLISM, CLINICAL AND EXPERIMENTAL RESEARCH; Waly,MI; 35(2):277-283 (2011)]. DNA methylation declines with age, resulting in cellular dysregulation. Low DNA methylation can lead to mutations and chromosome instability. Excessive SAMe methylation, on the other hand, has been shown to result in toxic methanol, formaldehyde, and formic acid in the brain [LIFE SCIENCES; Lee,E; 83(25-26):821-827 (2008)].
Although SAMe is the principle methyl donor in all cells of the body, SAMe is mainly synthesized, utilized, and degraded in the liver. Up to 80% of liver methionine is converted to SAMe, which is the source of glutathione, a principle antioxidant required for liver detoxificiation reactions [AMERICAN JOURNAL OF CLINICAL NUTRITION; Bottiglieri,T; 76(suppl):1151s-1157s (2002)]. Low SAMe levels in the liver associated with the removal of liver tissue is a stimulus for liver tissue regeneration [JOURNAL OF GASTROENTEROLOGY AND HEPATOLOGY; Lu,SC; 23(suppl 1):s73-s77 (2008)].
SAMe has also been shown to increase glutathione in forebrain tissue, inhibiting lipid peroxidation [NAUNYN-SCHMIEDENBERG'S ARCHIVES OF PHARMACOLOGY; De La Cruz,JP; 361(1):47-52 (2000)]. SAMe protects rat brains against oxidative stress associated with ischemia-reperfusion injury [BRAIN RESEARCH; Villalobos,MA; 883(1):31-40 (2000)].
SAMe has proven to be effective in the treatment of depression, with fewer side effects than is seen with conventional antidepressants [AMERICAN JOURNAL OF CLINICAL NUTRITION; Chiaie,RD; 76(suppl):1172s-1176s (2002) and AMERICAN JOURNAL OF PSYCHIATRY; Papakostas,GI; 167(8):942-948 (2010)]]. Nearly a third of severely depressed patients have folic acid deficiency accompanied by high plasma homocysteine [JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY; Bottiglieri,T; 69(2):228-232 (2000)].
SAMe can cause insomnia if not taken in the morning, and is best taken on an empty stomach.
|
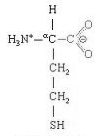
Homocysteine is an amino acid homolog of the amino acid cysteine, differing by having an additional methylene (−CH2−) group. SAH (S−Adenyl Homocysteine) produced from demethylation of SAMe is usually quickly transformed into homocysteine, which is more stable than SAH. High protein diets (which are high in methionine) result in significantly higher blood plasma levels of homocysteine than high carbohydrate diets [AMERICAN JOURNAL OF CLINICAL NUTRITION; Verhoef,P; 82(3):553-558 (2005)], but a comparable amount of free methionine added to low protein diet quadruples plasma homocysteine above that of a high protein diet [AMERICAN JOURNAL OF CLINICAL NUTRITION; Verhoef,P; 80(3):674-679 (2004)]. Free serine amino acid and free methionine together produce plasma homocysteine levels that are only one third the homocysteine levels produced by free methionine, apparently due to the ability of serine to increase both the transsulfuration and remethylation of homocyseine [Ibid.]. On average, people over age 85 have double the plasma homocysteine levels of people under age 40 [AMERICAN JOURNAL OF CLINICAL NUTRITION; Jacques,PF; 69(3):482-489 (1999)]. One study showed that 65% of vegans had moderate hyperhomocysteinemia compared to 45% of omnivores — and plasma B12 levels were considerably lower (although within normal range) [ANNALS OF NUTRITION & METABOLISM; Majchrzak,D; 50(6):485-491 (2006)].
High plasma homocysteine has been shown to compromise the blood-brain barrier in mice [BLOOD; Kamath,AF; 107(2):591-593 (2006)]. Homocysteine promotes atherosclerosis through fibrin deposition, oxidant stress, cytokine release, inflammation and other mechanisms [AMERICAN JOURNAL OF CLINICAL NUTRITION; McCully,KS; 86(suppl):1563S-1568S (2007)]. The atherosclerosis associated with high plasma homocysteine may also be partly due to homocysteine-induced stress to the endoplasmic reticulum of endothelial cells [THE JOURNAL OF CLINICAL INVESTIGATION; Werstuck,GH; 107(10):1263-1273(2001)]. Homocysteine damages endothelial cells [FEBS LETTERS; Xu,D; 470(1):20-24 (2000)]. Endothelial dysfunction has been shown to incrementally increase with incrementally higher doses of oral methionine (and subsequent incrementally higher plasma homocysteine) in normal human subjects [ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY; Chambers,JC; 19(12):2922-2927 (1999)]. Endothelial dysfunction subsequent to methionine administration was prevented by pretreatment with Vitamin E and Vitamin C in one study [JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION; Nappo,F; 281(22):2113-2118 (1999)], but not in another [JOURNAL OF HYPERTENSION; Tousoulis,D; 28(5):925-930 (2010)].
A double-blind study of high-dose folic acid, Vitamin B6, and Vitamin B12 administered for 24 months to persons over age 70 having mild cognitive impairment showed a 53% reduction in the rate of brain atrophy associated with reduced plasma homocysteine [PLOS ONE; Smith,AD; 5(9):e12244 (2010)]. Homosysteine administration impairs memory, and effect that can be prevented by the administration of antioxidant Vitamin E and Vitamin C [METABOLIC BRAIN DISEASE; Reis,EA; 17(3):211-217 (2002)]. Free radical inhibition of Na+/K+-ATPase activity by homocysteine is opposed by the antioxidant vitamins E & C [NEUROCHEMICAL RESEARCH; Wyse,ATS; 27(12):1685 (2002)].
Two large studies demonstrated that a combination of folic acid, Vitamin B6, and Vitamin B12 could lower plasma homocysteine, but did not reduce heart disease [NEW ENGLAND JOURNAL OF MEDICINE; 354(15):1567-1577 (2006) and 354(15):1578-1588 (2006)].
Although high plasma homocysteine has been associated with elevated risk of heart disease and stroke, the association is much stronger in persons who smoke or have high systolic blood pressure [JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION; Clarke,R; 288(16):2015-2022 (2002)]. Healthy young male subjects administered folic acid, Vitamin B6, and Vitamin B12 showed reduced plasma homocysteine, but no alteration in endothelial function [CLINICAL CARDIOLOGY; Hirsch,S; 25(11):495-501 (2002)]. Although B Vitamin therapy has been shown to reduce plasma homocysteine in clinical trials, little or no reduction in cardiovascular disease risk has been seen [ANNUAL REVIEW OF MEDICINE;Maron,BA; 60:39-54 (2009) and [FRONTIERS IN BIOSCIENCES; Lin,C; 14:3836-3845 (2009)].
|
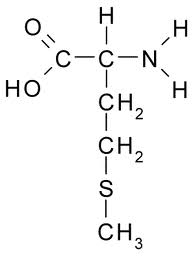
Methionine is the only essential amino acid containing sulfur. Methionine is the precursor of the other sulfur-containing amino acids: cysteine, taurine, homocysteine, and cystathione. Methionine is essential for the synthesis of proteins and many other biomoleules required for survival. Rats fed a diet without methionine develop fatty liver disease which can be corrected by methionine supplements [DIGESTIVE DISEASES AND SCIENCES; Oz,HS; 53(3):767-776 (2008)]. Dietary methionine is essential for DNA methylation. Reduced DNA methylation results in genetic instability, aberrant gene expression, and increased cancer — although high methionine intake may increase cancer in different ways than low methionine intake. Rats fed soybean protein supplemented with methionine had 40% more mammory tumors [JOURNAL OF NUTRITION; Hawrylewicz,EJ; 125(3 suppl):698S-708S (1995)].
Cereals (which are relatively high in methionine for a plant protein) can be combined with legumes (which are low in methionine) to provide a complete protein for vegetarian diets, despite the fact that it is unnecessary to do so [AMERICAN JOURNAL OF CLINICAL NUTRITION; Young,VR; 59(suppl):1203s-1212s (1994)]. Although methionine is required for protein synthesis, nearly half of dietary methionine is converted to SAMe in the liver [THE FASEB JOURNAL; Lu,SC; 13(10):1169-1183 (1999)].
Rats fed a diet containing about 20% of the normal amount of methionine showed a reduction in serum levels of all sulfur-containing amino acids, with the exception of homocysteine, which was paradoxically increased to 2½ times control levels [NUTRITION; Elshorbagy,AK; 26(11-12):1201-1204 (2010)]. High dietary methionine is the typical means of elevating serum homocysteine, but both high and low levels of methionine can increase serum homosysteine levels [ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY; Holm,PI; 25(2):379-385 (2005)]. Rats fed toxic high levels of methionine could be protected from methionine toxicity by simultaneous dietary supplementation with glycine and serine [ARCHIVES OF BIOCHEMISTRY AND BIOPHYSICS; Regina,M; 300(2):588-607 (1993)].
Atherosclerosis-prone mice (apo-E deficient mice) fed an experimental diet that elevated blood plasma methionine without elevating plasma homocysteine showed considerable atherosclerosis, whereas the mice fed B Vitamin-deficient diets developed severely elevated homocysteine without atherosclerosis [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Troen,AM; 100(25):15089-15094 (2003)]. A similar experiment on atherosclerosis-prone mice showed toxic effects of methionine on endothelial cells [EXPERIMENTAL AND MOLECULAR PATHOLOGY; Aleisso,ACM; 90(1):45-50 (2011)]. A high methionine diet in normal ("wild-type") mice was shown to reduce serum HDL-cholesterol by decreasing HDL-cholesterol production [JOURNAL OF NUTRITIONAL BIOCHEMISTRY; Velez-Carrasco,W; 19(6):362-370 (2008)].
Fruit flies fed triple the normal amount of methionine have 10% shorter mean lifespan [AGE; Troen,AM; 29(1):29-39 (2007)]. Rats fed extra methionine show elevated iron and lipid peroxidation in the liver [JOURNAL OF NUTRITION; Mori,N; 130(9):2349-2355 (2000)] and advanced vascular disease (intima thickening) [JOURNAL OF NUTRITION; Fau,D; 118(1):128-133 (1988)].
(See also Life Extension Benefits of Methionine Restriction)
|
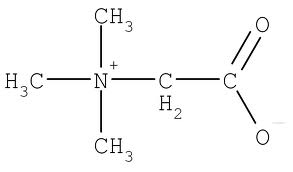
Betaine (TMG, TriMethylGlycine) protects plants and shellfish from osmotic deactivation due to drought or conditions of high salinity. Thus, TMG can be obtained in the diet from shellfish and certain plants. TMG is particularly high in wheat bran and wheat germ, but it is also high in spinach, as well as in beets (where it was first discovered). TMG is derived from choline in rats, but this does not happen much in humans [AMERICAN JOURNAL OF CLINICAL NUTRITION; Lieber,CS; 76(suppl):1148s-1150s (2002)], who are dependent upon dietary sources.
In the kidney, TMG osmotically protects cells from urea and high electrolyte concentrations. In the liver, TMG protects against chloroform and carbon tetrachloride toxicity — as well as against methyl depletion by excess niacin catabolism [AMERICAN JOURNAL OF CLINICAL NUTRITION; Craig,SAS; 80(3):539-549 (2004)]. About half of diabetic subjects and more than half of obese subjects suffer from fatty liver, a condition that can be significantly reduced by TMG [Ibid.].
TMG converts homocysteine to methionine in the liver, whereas folate-dependent remethylation of homocysteine takes place in all cells. TMG strongly reduces plasma homocysteine in subjects with low serum folate, and has a weaker effect when folate is high, whereas Vitamin B6 has little or no effect on plasma homocysteine levels [ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY; Holm,PI; 25(2):379-385 (2005)]. TMG has little effect on plasma homocysteine in healthy subjects [EUROPEAN JOURNAL OF CLINICAL NUTRITION; Schwab,U; 65(1):70-76 (2011)], but can protect against the elevated homocysteine resulting from feeding methionine [NUTRITION, METABOLISM, AND CARDIOVASCULAR DISEASES; Atkinson,W; 19(11):767-773 (2009)].
Much of the SAMe in the liver is converted to the water-phase antioxidant glutathione. Six grams of TMG daily for three months reduced plasma homocysteine levels by 9% in 22 obese subjects [AMERICAN JOURNAL OF CLINICAL NUTRITION; Schwab,U; 76(5):961-967 (2002)]. TMG decreases plasma homocysteine more in patients with high plasma homocysteine than in healthy volunteers [ARCHIVES OF INTERNAL MEDICINE; Brouwer,IA; 160(16):2546-2547 (2000)]. Because TMG helps reduce homocysteine, it could protect against heart disease — but see the sections above on folic acid and Vitamin B12.