Research devoted to cryopreservation of organs is rare, but research devoted entirely to the objectives of cryonics is even more rare. Although there is much to be learned from cryobiologists, especially cryobiologists dedicated to organ preservation, the different objectives of cryonics may require different methods.
The objectives of organ cryopreservationists is to maximize viability, ie, to maximize living functionality of organs rewarmed from cryopreservation. By contrast, the objective of cryonics research is to preserve organs — especially the brain — in such a way as to optimize restoration by future technology. (Admittedly, this involves second-guessing the capabilities of future technology.)
If, for example, cryopreservation method A resulted in 60% viability & 80% structural preservation, whereas method B resulted in 50% viability & 90% structural preservation — the organ cryopreservationist would prefer method A, whereas the cryonicist might prefer method B. Normally, good structure leads to good function, but it is possible that (for example) methods to minimize damage upon rewarming could entail methods that are suboptimal for structural preservation on cooling.
Vitrification requires the use of cryoprotective agents ("antifreeze compounds") to prevent ice formation and/or freezing damage by increased viscosity, by hydrogen-bonding with water molecules, by dilution of electrolytes and/or by colligative interference. Ice formation is a two-step process involving first the formation of a nucleus (nucleation) and second the growth of an ice crystal from the nucleus.
Cell membranes can be called semi-permeable membranes because they allow some ions & molecules to pass through more readily than others — while some molecules cannot pass through at all. Both chemical concentrations and ionic concentrations must reach equilibrium (electrical equilibrium for concentrations of ions). Some ions and molecules are actively transported at the expense of cellular energy. Cells contain many large negatively charged molecules (anions, A-) — mostly proteins, amino acids and organic phosphates — which are too large to pass through the cell membrane. Cell membranes are also normally impermeable to the positive calcium (Ca2+) ions outside the cell.
Slow leak channels allow potassium (K+), sodium (Na+), and chlorine (Cl-) ions to cross the cell membrane. Both K+ and Na+ have a strong electrical attraction to the anions in the cell, but the slow leak channels allow K+ to enter the cell at 100 times the rate that Na+ can enter. Although Na+ (atomic number 11) is a smaller ion than K+ (atomic number 19), the more concentrated positive charge causes Na+ to surround itself with more tightly-held water molecules than K+. The hydrated diameter of Na+ is 5 Angstroms, whereas the hydrated diameter of K+ is only 3.8 Angstroms. This allows hydrated K+ to pass through the membrane slow-leak channels far more easily than Na+.
Normal concentration of Na+ outside of a cell is typically 10 times that found inside a cell, whereas K+ concentration is typically 20-35 times greater inside than outside. At equilibrium, the strong electrical attraction which draws K+ into a cell is equal to the force of the concentration gradient which would tend to cause K+ to leave a cell.
The Na+ and K+ ions that enter a cell are insufficient to neutralize the negative charge of the anions inside the cell. Because of this, animal cells have a net negative charge inside. Animal membranes act like capacitors, with a negative charge on the inside and a positive charge on the outside of the membrane. This voltage difference across the membrane is called the membrane potential. The resting membrane potential of large mammalian nerve fibers is about −90 milliVolts (mV, negative inside the membrane), whereas the giant squid axon (which is an excellent model for experiments) has a membrane potential of −60 mV.
The Nernst Equation provides a means of calculating the membrane potential which would be produced for a single ion having relatively different concentrations on either side of a membrane. For example, an out/in ratio of 20/400 for K+ gives a Nernst potential of −75 mV at 25ºC. For Na+ with an out/in ratio of 440/50 there would be a Nernst potential of +55 mV. These figures (ECF=ExtraCellular Fluid, ICF=IntraCellular Fluid) correspond to what is found in the membrane of a giant squid axon:
ION |
ECF (mM) |
ICF (mM) |
Nernst Potential (mV) |
|---|---|---|---|
| K+ | 20 | 400 | -75 |
| Na+ | 440 | 50 | +55 |
| Cl- | 560 | 52 | -60 |
| A- | -- | 385 | -- |
The membrane potential will be the result of all of the ions, calculated by the Goldman Equation (also known as the Goldman-Hodgkin-Katz Equation). The Goldman Equation looks much like the Nernst Equation with cation concentrations in the numerator, except that the concentrations are multiplied times permeabilities to give a factor that has been called conductance. Because K+ has such a high relative permeability, it dominates the Goldman Equation, causing the resulting potential to be nearly the same as the potential predicted by the Nernst Equation for K+ — which is, in fact, what the resting membrane potential approximates. When an axon depolarizes in an action potential, however, a voltage-gated Na+ channel opens and the Na+ permeability temporarily becomes far greater than that for K+, the Na+ term dominates the Goldman Equation and the membrane potential is temporarily close to the Nernst potential for Na+.
The resting membrane potential of the giant squid axon is −60mV, equal to the Nernst potential of Cl-. In fact, Cl- passively equilibrates to match the membrane potential which is determined by the other ions and forces — which is why Cl- can be ignored (dropped from the Goldman Equation) when calculating membrane potential.
The membrane potential is maintained by the so-called sodium pump — a membrane enzyme (Na/K-ATPase) which uses one molecule of ATP to eject 3 Na+ ions out of a cell while bringing 2 K+ ions into the cell — a net ejection of one positive ion. The sodium pump is essential for maintaining membrane potential.

|
The sodium pump will not function without binding to ATP & Na+ inside the membrane and K+ outside the membrane. The poison ouabain binds to the K+ receptors of the sodium pump outside the membrane, thereby inactivating the sodium pump. Although a cell can maintain a membrane potential for several hours without a functional sodium pump, the slow leak of Na+ into the cell and consequent leak of K+ out of the cell will result in a complete loss of membrane potential after several hours. Similarly, if the cell dies in the sense of no longer being capable of producing energy (ATP) in the mitochondria, the sodium pump will cease to operate. Thus, normal intracellular K+/Na+ ratios can be used as an index of viability.
Intracellular K+/Na+ ratios is the most commonly used method of assessing viability in cryonics research, although other methods (such as measurement of intracellular ATP content) could be useful in the future. To assay the intracellular K+/Na+ ratio tissues are placed in mannitol to wash away extracellular ions. Then TriChloroAcetic acid is used to rupture cell membranes and release intracelluar ions. A spectrophotometer can be used to determine the relative concentrations of sodium and potassium ions in the cells.
Most people are unaware that the temperature at which pure water (homogenous water) will freeze is much lower than the temperature at which pure water will melt. Thermal hysteresis exists when there is a difference between freezing and melting temperature — which can result in different effects of warming and cooling over the temperature range between freezing and melting, depending on the thermal history.
The reason tapwater freezes at just below 0ºC — close to the temperature at which it melts (Tm = 0ºC) — is because of impurities that serve as nucleating agents. Tiny water droplets in clouds at an altitude of one to eight miles can be −15ºC or colder and still will not freeze without the presence of a nucleator (eg, a particle of mineral dust). (The white of clouds is due to the fact that — as with milk — the droplets are roughly the size of the wavelength of visible light. Scattered light appears white.)
Water that is perfectly pure is unlikely to freeze at −5ºC because an ice crystal cannot grow at that temperature without achieving a critical crystal nucleus mass of 45,000 water molecules. With fewer than 45,000 water molecules the nucleus is dissolved because heat of fusion warms the nucleus too much and the dissolving effect of the surrounding medium is too great. The critical size required for crystal growth in pure water at −20ºC is 650 water molecules, far more molecules than would be expected to aggregate at that temperature in human-scale volumes and times. Pure water freezes at about −40ºC (Th, homogenous freezing temperature) when a nucleus need only contain 70 water molecules to be large enough to grow. (For more on the nucleation process, see Some Properties of Metals.)
Biological nucleating agents in the environment are the most common reason that water freezes just below 0ºC rather than at −40ºC. When water nuclei grow around a non-water nucleus such as silver iodide (AgI) or a bacterial protein, water freezes by heterogenous nucleation at a heterogenous freezing temperature. The bacteria Pseudomonas syringe commonly found on plants can elevate freezing temperature of water to a heterogenous freezing temperature of −2ºC. Many organisms (such as arctic insects and fish) have anti-freeze proteins which inhibit ice nucleation, thereby lowering the heterogenous freezing temperature.
The melting temperature of water (Tm), the freezing temperature of water which contains no nucleating agents (Th), and the heterogenous freezing temperature (temperatures between Tm and Th, inclusive of the former) are all lowered by the addition of cryoprotective agents. If enough cryoprotective agent is added the freezing temperature Th becomes so low that it reaches Tg, the temperature at which the water-cryoprotectant mixture forms a glass (vitrifies) rather than freezes into ice crystals [CRYOBIOLOGY 21(4):407-426 (1984)]. Tg is typically about −123ºC for cryoprotectants used in cryonics.
(For more details on melting, homogenous and heterogenous nucleation temperatures, see ICE BLOCKERS.)
|
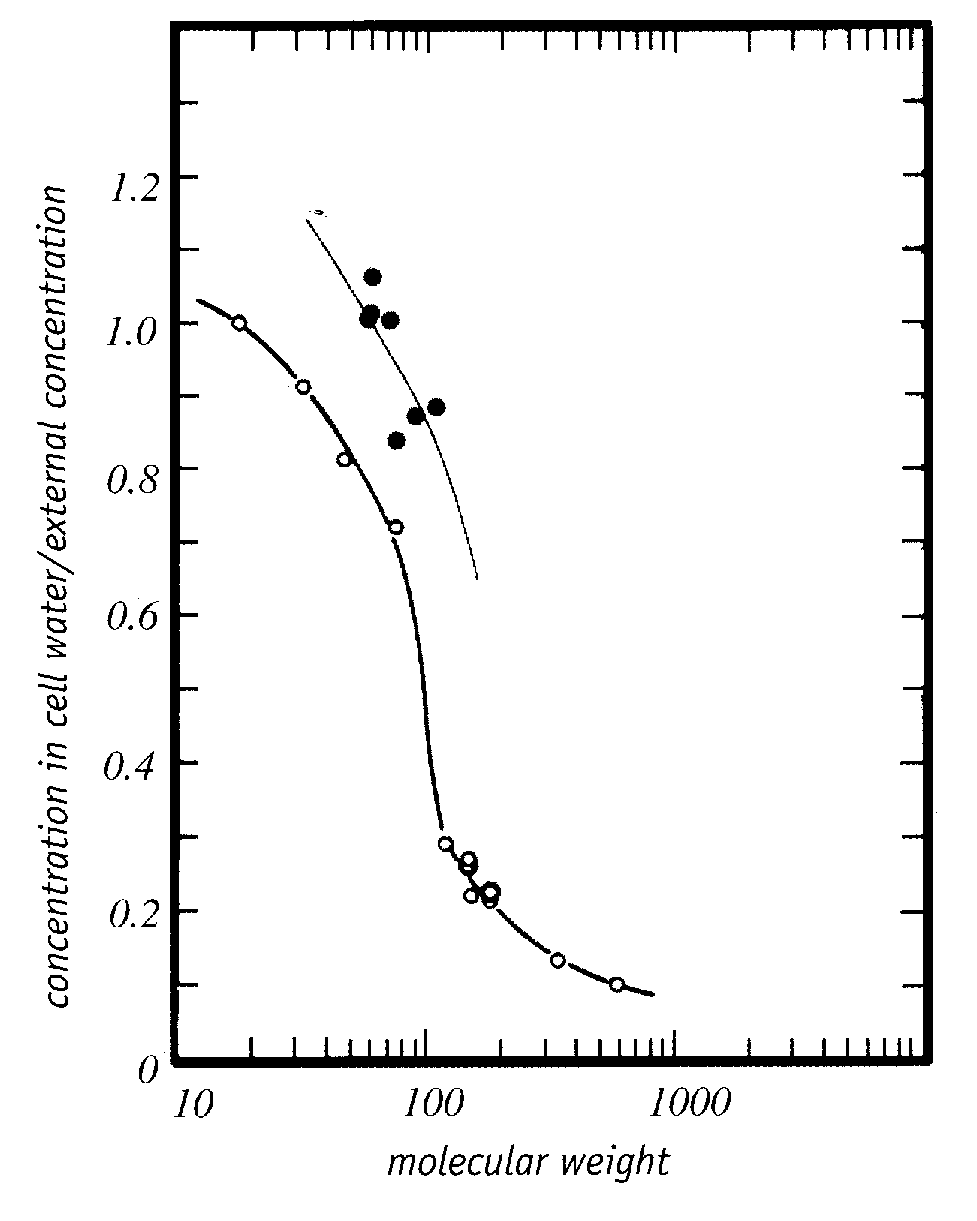
At least 90% of the water in a cell is what can be called "bulk water". Bulk water is the water that can freeze and/or move out of the cell in response to changes in osmotic pressure (e.g., by a hypertonic solution outside the cell, which could be due to the formation of extracellular ice). By contrast, about 10% of cell water is bound water that is tightly hydrogen-bonded to the hydrophilic surfaces of macromolecules (proteins, nucleic acids or the polar end-groups of phospholipids) — or water molecules that are tightly hydrogen-bonded to the water molecules directly bound to the macromolecules [ANNALS OF THE NEW YORK ACADEMY OF SCIENCES; Despa,F; 1066:1-11 (2005)]. Bound water does not osmotically leave the cell due to extracellular freezing or other causes of extracellular hypertonicity. Bound water can only be removed by heating in a vacuum. Removal of bulk water does not cause dehydration damage, but removal of bound water does cause such damage — because macromolecules chemically interact when they do not have the protective covering provided by bound water.
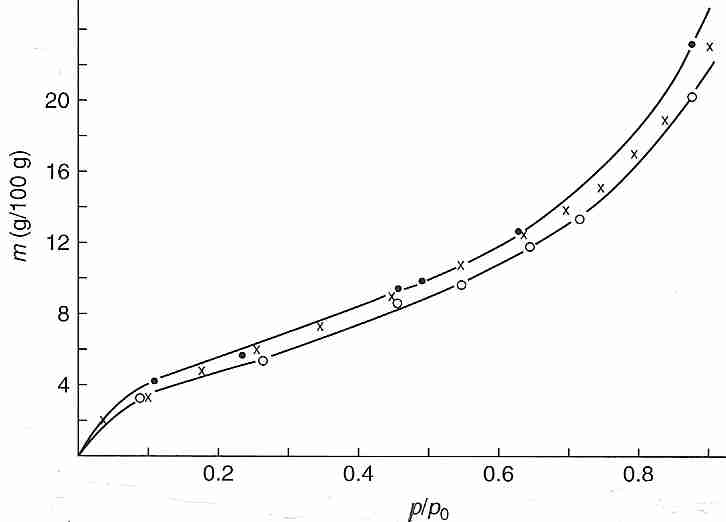
Bound water can be experimentally demonstrated by the vapor pressure (p) measured at 25ºC over bovine hemoglobin (native, X, or denatured, O) that has been dehydrated by varying degrees of oven drying. Plotting the water content (grams water per 100 grams of hemoglobin, the independent variable as the vertical axis) against the ratio of dehydrated vapor pressure to vapor pressure of pure water (p/po) demonstrates that above about 20% (20 g/100 g) water, the vapor pressure is nearly the same as that of pure water (p/po > 0.8) — meaning that most of the water is bulk water not bound to protein. At about 10% water content p/po has dropped to about half (0.5) that of pure water, which can be taken to indicate that the remaining water in the system is even more tightly bound to the protein. Calorimetry experiments on a number of protein solutions verify that there is no latent heat of fusion (no freezing) for 25%-30% of the water — meaning that the water is bound to the protein. Bound water is so viscous that it vitrifies at cryogenic temperatures. Differential Scanning Calorimeter (DSC) measurements of cells subjected to cooling also indicate that at least 10% of cell water is incapable of freezing [CRYOBIOLOGY; Schrenders,PD; 33(5):487-501 (1996) and CRYOBIOLOGY; Sun,WQ; 38(4):372-385 (1999)].
![[ Clathrate Hydrate containing Guest Molecule]](clath1.jpg)
|
![[ Clathrate Hydrate containing Guest Molecule ]](clath0.jpg)
|
Water can be incorporated into the crystals of many inorganic and organic molecules giving hydrate forms. Protein crystals are commonly 50% water. But many nonpolar molecules and polar molecules having nonpolar portions (such as proteins) can also be dissolved in water without any ionic or hydrogen bonding with the water molecules. Nonpolar groups in proteins, such as the methyl group of alanine, the benzyl group of phenylalanine, or the isopropyl group of valine are often driven to the interior of proteins (protein folding) by hydrophilic interaction with water. Even so, nearly half of the nonpolar groups in proteins may be found on the surface area (hydrated area). As temperature drops, hydrophobic interactions become weaker and hydrogen bonds become stronger.
Water molecules form a cage-like structure clathrate hydrate around the nonpolar "guest molecules". The clathrate hydrate is characterized by hydrogen bonds between the water molecules forming the cage and van der Waals interactions between the water molecules and the guest molecules in the cage.
Gases such as hydrogen sulfide and methane are often maintained
in water solutions as clathrates, especially under high pressure.
More than 6 trillion tons of methane clathrate can be found
in the deep ocean floor. Methane can also act as a "helper
molecule" that results in alcohols normally soluble in
water (such as isopropyl alcohol) forming clathrate
hydrates [THE JOURNAL OF CHEMICAL PHYSICS; Alavi,S; 133(7):074505 (2010)].
Methanol, by contrast, is a hydrate inhibitor which has been used
in gas pipelines to prevent the formation of hydrates which can
impede flow. Other alcohols can be either guest molecules or
clathrate inhibitors (depending on temperature and pressure) due to
hydrogen bonding between the guest molecule oxygen and the water
molecules in the
cage [CHEMISTRY:A EUROPEAN JOURNAL; Alavi,S; 16(3):1017-1025 (2010)
and PHYSICAL CHEMISTRY CHEMICAL PHYSICS; Makiya,T; 12(33):9927-9932 (2010)].
![[ Polyhedral crystalline array]](clath2.jpg)
|
The cage-like structure of polyhedral clathrates around guest molecules is a crystalline-type structure resembling ice and possessing a defined melting point. The structure of methane clathrate hydrate in liquid water is essentially the same as its structure in ice — evidenced by the fact that the solubilization heat (ΔHº) for methane incorporation in liquid water (−4621 calories) is virtually identical to that of ice (−4553 calories). Clathrate polyhedra unit cells can share faces to build into a macroscopic crystalline array. Higher pressures alter hydrate crystal morphology leading to hydrate crystals that grow into liquid water from a hydrate film [CRYSTAL GROWTH & DESIGN; Ohmura,R; 5(3):953-957 (2005)]. The volume of a clathrate is greater than that of hexagonal ice containing the same number of molecules — even excluding the guest molecule. For this reason, clathrate formation in biological tissues could be expected to cause more mechanical damage than freezing. On the other hand, by preventing phase separation of water molecules into pure ice, clathrates (such as xenon) could prevent damage due to high salt solute concentration.
A paper on mouse hearts subjected to xenon-nitrogen at 100 psi (6.8 atmospheres) pressure, reportedly shows good electron micrographs of cardiomyocytes that had been in liquid nitrogen compared to controls subjected to oxygen-nitrogen under pressure [INTERNATIONAL JOURNAL OF CLINICAL AND EXPERIMENTAL PATHOLOGY; Sheleg,S; 1(5):440-447 (2008)]. The paper suggests that clathrates formed in the cell reduce the amount of extracellular ice by retaining water in the cell that would otherwise have migrated across the cell membranes during the freezing process, thereby protecting the cardiomyocytes from dehydration damage. The paper also suggests that the xenon clathrates are protecting cells from recrystallization damage during the thawing process. If the latter explanation is true, then the improved micrographs are not indicative of improved structural preservation during cryostasis. The paper is critical of vitrification because of cryoprotectant toxicity and another vaguely-referenced source of injury (possibly devitrification). This is given as a reason why a vitrified sample was not prepared for comparison. Vitrification and clathrate formation are incompatible approaches to cryopreservation. Clathrates are structures similar to ice, which means that vitrification would prevent clathrate formation at least as readily as it prevents ice formation. Although xenon clathrates may be useful for cryogenic temperature cryopreservation of small tissue samples, it cannot be applied to cryogenic organ cryopreservation or cryonics because of the mechanical damage caused by the large-volume clathrates.
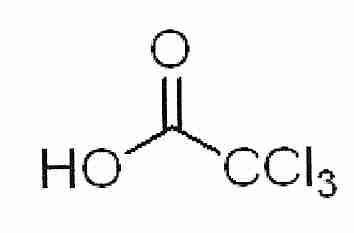
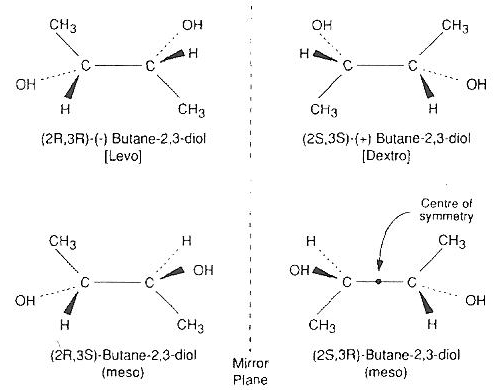
2,3−butanediol is a powerful cryoprotectant that can vitrify at lower concentrations than a number of other cryoprotectants. 2,3−butanediol has meso isomers that form a hydrate that readily crystallizes and is cytotoxic [CRYOBIOLOGY; Sutton,RL; 29(5):585-598 (1992)]. Whether the crystallization is responsible for the cytotoxicity — and whether the hydrates are nucleating ice — remains to be seen. Pure levo or dextro 2,3−butanediol (which does not include the meso isomers) is very expensive, although 2,3−butanediol solutions with less than 4% meso isomers are not so expensive.
| Polyol CPAs | DMSO |
|---|---|
![[Polyol CPA structures ]](polyols.jpg)
|
![[ DMSO ]](DMSO.jpg)
|
Glycerol can cause kidney failure by increased hydrogen peroxide generation [AMERICAN JOURNAL OF PHYSIOLOGY; Guidet,B; 257(3 Pt 2):F440-F445 (1989)] and increased nitric oxide release [RENAL FAILURE; Chandler,V; 28(2):161-168 (2006)].
Ethylene glycol is metabolized to glycoaldehyde, glyoxylate and oxalic acid [AMERICAN JOURNAL OF KIDNEY DISEASES; Poldelski,V; 38(2):339-348 (2001)]. Toxic metabolites of ethylene glycol can cause kidney and cardiopulmonary failure [FORENSIC SCIENCE INTERNATIONAL; Leth,PM; 155(2-3):179-184 (2005)]. Glycoaldehyde has also been linked to skin damage [ARCHIVES OF TOXICOLOGY; Lockley,DJ; 79(3):160-168 (2005). Antioxidants have been shown to reduce the toxic effects of kidney epithelial cells exposed to oxalate and calcium oxalate [UROLOGICAL RESEARCH; Thamilselvan,S; 31(1):3-9 (2003)]. Some investigators claim that renal toxicity is only due to the calcium oxalate [TOXICOLOGY LETTERS; Guo,C; 173(1):8-16 (2005)]. For mouse oocytes, removal of calcium opposes the toxicity of ethylene glycol [MOLECULAR REPRODUCTION AND DEVELOPMENT; Takahashi,T; 68(2):250-258 (2004)], DMSO [REPRODUCTION; Larman,MG; 131(1):53-61 (2006)] and propylene glycol [HUMAN REPRODUCTION; Larman,MG; 22(1):250-259 (2007)].
But cryoprotectant toxicity in cryobiology (and cryonics) is not related to long-term, high-temperature systemic effects — particularly liver breakdown products like ethylene glycol metabolites. (Ethylene glycol itself has low systemic toxicity). How toxicity is assayed influences how toxicity is explained. K/Na ratio is a commonly used assay for viability or toxicity (toxicity is high when viability is low).
For kidney slices glycerol is generally the least toxic of the of the conventional CryoProtectant Agents (CPAs), which can be ordered by toxicity as:
formamide > propylene glycol > DMSO > ethylene glycol > glycerol
With the exception of formamide, this is the exact order of the vitrifying capability of these CPAs, with glycerol being both the least powerful CPA and the least toxic [CRYOBIOLOGY; Baudot,A; 40(2):151-158 (2000)]. Formamide is the most toxic CPA, but has the least glass-forming ability (it cannot vitrify by itself, but can assist vitrification by other cryoprotectants). When formamide and the two glycols are combined with DMSO, the formamide-DMSO combination is the least toxic — DMSO considerably reduces formamide toxicity (and apparently vice-versa, see next section). The order of toxicity in this case is:
DMSO+propylene glycol > DMSO+ethylene glycol > DMSO+formamide
A study of mouse blastocysts [ JOURNAL OF REPRODUCTION AND FERTILITY; Valdez,CA; 96(2):793-802 (1992)] using 30%(v/v) of six cryoprotectants found Ethylene Glycol (EG) to be by far the least toxic and Propylene Glycol (PG) nearly the most toxic:
1,3-butanediol > PG > 2,3-butanediol > glycerol > DMSO > EG
For human and mouse oocytes, however, DMSO may be more toxic than propylene glycol [HUMAN REPRODUCTION; Gook,DA; 8(7):1101-1109 (1993)]. And for human endothelial cells, even at 2-4ºC DMSO and ethylene glycol are far more toxic than propylene glycol or 2,3-butanediol [CRYOBIOLOGY; Wusterman,MC; 44(1):24-37 (2002)].
Conversely, DMSO results in much less damage to buffalo oocytes recovered from slow freezing than does ethylene glycol or propylene glycol. Vitrified buffalo oocytes were about 40% more likely to become blastocyts with 40% DMSO than with 40% ethylene glycol, but more than 3 times more likely with 20% DMSO and 20% ethylene glycol [REPRODUCTION, FERTILITY, AND DEVELOPMENT; Gautam,SK; 20(4):490-496 (2008)].
Similarly, for oyster embryos, DMSO is less toxic than propylene glycol, ethylene glycol or acetamide. And the combination of DMSO with acetamide is less toxic than either DMSO or acetamide alone. Addition of the sugar trehalose further reduces toxicity and increases survival [AQUATIC LIVING RESOURCES; Chao,N; 7(2):99-104 (1994)].
For flounder embryos, the order of toxicity for 20% CPA concentrations is:
glycerol = ethylene glycol > DMSO > DimethylFormamide > propylene glycol > methanol
No flounder embryos survive exposure to ethylene glycol or glycerol [THERIOGENOLOGY; Chen,SL; 63(4):1207-1219 (2005)]. Flounder embryos showed a significant reduction in the cryoprotectant toxicity of glycerol, DMSO, propylene glycol and ethylene glycol by the addition of 5% methanol [THERIOGENOLOGY; Zhang,YZ; 63(3): 763-773 (2005)].
For zebrafish embryos, the order of toxicity for a 10% cryoprotectant solution with 48 hours of exposure is:
ethylene glycol > methanol > propylene glycol > DMSO
from [THERIOGENOLOGY; Lahnsteiner,F; 69(3):384-396 (2008)]
For penaeid prawn embryos, the order of toxicity of cryoprotectant solutions is
glycerol, formamide > propylene glycol > DMSO > ethylene glycol > methanol
where glycerol was more toxic at the morula stage, but formamide was more toxic at the nauplii state [CRYOBIOLOGY; Newton,SS; 33:172-177 (1996)].
For Escherichia coli bacteria DiEthylSulfOxide (DESO) is considerably less toxic than DMSO or glycerol [CRYOBIOLOGY; Markarian,SA; 49(1):1-9 (2004)]:
glycerol > DMSO > DESO
For Chlamydomonas reinhardtii alga, photosynthetic activity (as a viability assay) was reduced to nearly 25% by 7.5% DMSO, but was nearly normal for 7.5% methanol [EUROPEAN JOURNAL OF PHYCOLOGY; Crutchfield,ALM; 34(1):43-52 (1999)].
Variable results have been obtained when comparing DMSO with glycerol toxicity for different organisms. DMSO is more toxic than glycerol for chicken spermatazoa, assessed on the basis of fertility [POULTRY SCIENCE; Tselutin,K; 78(4):586-590 (1999)]. The diflagellate protozoan Crypthecodinium cohnii shows much better growth after exposure to glycerol than to DMSO [CRYOBIOLOGY; Simione,FP; 14(3):362-366 (1977)]. (Ethylene glycol was extremely toxic to these protozoans.) By contrast, the spirochete bacterium Leptospira interrogans shows much more toxic effect on growth with 2% glycerol than with 3% DMSO [THE JOURNAL OF APPLIED BACTERIOLOGY; Palit,A; 61(5):407-411 (1986)]. Glycerol is more toxic than DMSO to Plasmodium chabaudi malaria parasites, although distinguishing osmotic effects from actual toxicity complicates the comparison [CRYOBIOLOGY; Mutetwa,SM; 21(3):329-339 (1984)]. Addition of glucose to the recovery medium significantly improved recovery following exposure to both cryoprotectants. A 50% glycerol solution is less toxic to plant (Metha) mint shoot tips than 30% glycerol in combination with 15% ethylene glycol and 15% DMSO [CRYOBIOLOGY; Volk,GM; 52(2):305-308 (2006)].
Methanol and polyvinylpyrrolidone (PVP) are considerably less toxic to methanotropic bacteria than glycerol or DMSO [LETTERS IN APPLIED MICROBIOLOGY; Green,PN; 14(4):158-162 (1992)]. The superiority of methanol & ethanol over other cryoprotectants in cryopreservation of rapidly cooled Saccharomyces cerevisiae yeast is attributed to increased membrane permeability rather than reduced toxicity [CRYOBIOLOGY; Lewis,JG; 31(2):193-198 (1994)].
Rather than assess relative cryoprotectant toxicity in equal absolute concentrations, one can compare toxicity of cryoprotectants to endothelial cells at the concentrations needed to vitrify. Only 32% w/w 2,3−butanediol will vitrify endothelial cells, but for DMSO, ethylene glycol and propylene glycol, a 45% w/w concentration is required. Comparing endothelial cell toxicities at concentrations needed to vitrify gives:
DMSO = ethylene glycol (45%) > propylene glycol (45%) = 2,3−butanediol (32%)
which makes 2,3−butanediol (ordinarily considered the most toxic) less toxic to endothelial cells than DMSO or ethylene glycol [PHYSICS IN MEDICINE AND BIOLOGY; Robinson,MP; 47(13):2311-2325 (2002)]. Reducing 2,3-butanediol concentration from 3.0 Molar to 2.0 Molar has cut endothelial cell loss by a factor of 35, whereas the same molar reduction of DMSO has only cut endothelial cell loss by a factor of 3 [CRYOBIOLOGY; Bourne,WM; 31(1):1-9 (1994)].
|
2,3−butanediol nominally has four isomers, but two are identical meso isomers that form a hydrate that readily crystallizes and is cytotoxic [CRYOBIOLOGY; Sutton,RL; 29(5):585-598 (1992) and ORGANOGENESIS; Taylor,MJ; 5(3):155-166 (2009)]. Pure levo/dextro 2,3−butanediol containing no meso isomer is prohibitively expensive for most applications. The addition of 4% trehalose or sucrose to a 30% 2,3−butanediol solution that is 96.7% optically active (non-meso) isomers drastically reduces toxicity and cuts the cooling rate in half [CRYOBIOLOGY; Bouton,P; 31(4):367-373 (1994)] although adding 4% saccharide is only about half as effective at reducing cooling rate as adding 4% more 2,3−butanediol [CRYOBIOLOGY; Baudot,A; 33(3):363-375 (1996)]. Use of a carrier solution such as Euro-Collins can also reduce toxicity and increase glass-forming ability.
Many cryobiologists operate as though different toxicity rules apply to different cells, tissues, organs and organisms — limiting their focus to their own specialties. A Unified Theory of Cryoprotectant Toxicity must be possible, and is should be the sought in order to have the deepest understanding of mechanisms. Plants, oocytes, and fish embryos are composed of proteins, lipids, carbohydrates and nucleic acids no less than mammalian organs. Studying and understanding a wide variety of organisms and tissues provides the best potential for learning what is general and what is specific about CPA toxicity — which must ultimately come down to the interactions of CPAs with proteins, lipids, carbohydrates, nucleic acids, and water as well as interactions of CPAs.
Listing CPAs in order of viscosity gives:
glycerol > propylene glycol > ethylene glycol > DMSO
|
A good mixture of cryoprotectants will have low viscosity, low toxicity and good vitrifying capability. Reduced enzyme activity due to viscous cryoprotectant should not be equated with toxicity [BIOLOGICAL PROCEDURES ONLINE; Uribe,S; 5(1):108-115 (2003)].
Unlike the polyols (glycerol, ethylene glycol, propylene glycol, etc.), the
toxicity of DMSO can be reduced by mixing with other CryoProtectant Agents (CPAs).
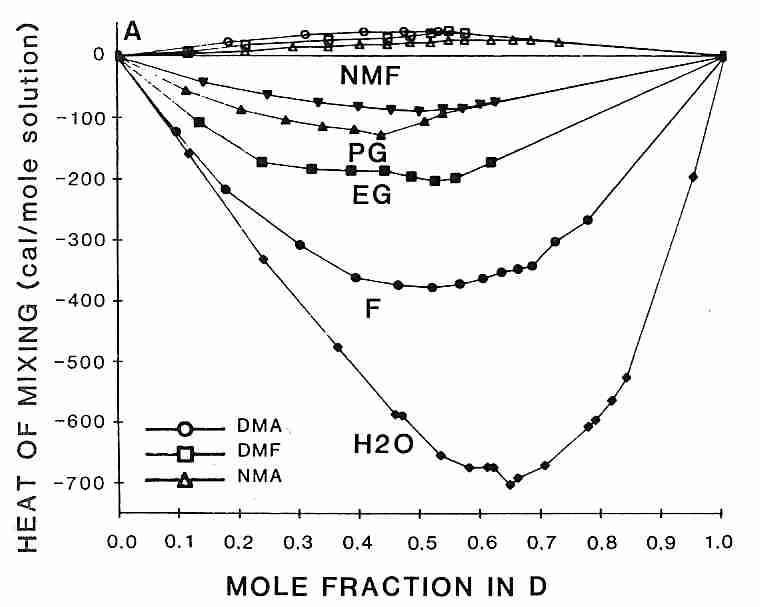
Interestingly, the rank order for heat-release-on-mixing of DMSO with other cryoprotectants
is the same as the order of effectiveness of reducing DMSO toxicity
[from CRYOBIOLOGY; Fahy,GM; 24(3):196-213 (1987)]:
formamide > ethylene glycol > propylene glycol > N-methyl formamide
probably because associating more strongly with DMSO corresponds with reducing its toxicity. Methylation of polyols increases glass-forming ability while increasing toxicity due to increased hydrogen bonding strength of the hydroxyl groups [JOURNAL OF PHYSICAL CHEMISTRY; Forsyth,M; 94:6889-6893 (1990)].
Not only do cryoprotectants diffuse through cell membranes at different rates, but they
affect the rate at which water molecules diffuse through membranes. Glycerol & propylene glycol
diffuse through human sperm membranes nearly two-thirds faster than DMSO, but water diffusion
through sperm membranes is nearly two-thirds faster with propylene glycol than with glycerol
or DMSO [BIOLOGY OF REPRODUCTION; Gilmore,JA; 53(5):985-995 (1995)].
|
Trehalose is a non-toxic sugar cryoprotectant which displaces bound water and protects cell membranes by hydrogen-bonding to proteins and the polar ends of phospholipids more strongly than bound water [ARCHIVES OF BIOCHEMISTRY AND BIOPHYSICS 245(1):134-143 (1986)]. Toxic cryoprotectants could prevent dehydration damage by the same means, while nonetheless causing protein denaturation which the more weakly hydrogen-bonding trehalose does not. There is evidence that good cryoprotectants are those that are preferentially excluded from proteins — thereby stabilizing them and opposing their unfolding or denaturation [ADVANCED DRUG DELIVERY REVIEWS; Arakawa,T; 46(1-3):307-326 (2001)]. Such cryoprotectants would be hydrophobic enough to cross cell membranes readily — cryoprotectants cross cell membranes far more readily than would be predicted by their molecular weight [Figure 5, PHYSIOLOGICAL CHEMISTRY AND PHYSICS AND MEDICAL NMR 25:177-208 (1993)] — and would be hydrophilic enough to readily mix with water. Less toxic cryoprotectants may act more by colligative interference with ice formation than by hydrogen-bonding with water.
Understanding the molecular mechanisms of the toxicity of cryoprotectant agents would be an important step toward finding means of reducing toxicity. Cryoprotectants can be toxic in different ways, but there are apparently common features of toxicity which correlate with the capacity of substances to cryoprotect. In some instances cryoprotectant toxicity can be confused with osmotic damage. Cryoprotectants and their carrier solutions must protect against osmotic damage, chilling injury, protein damage, and damage to a variety of cellular organelles and tissues which can be associated with both cooling and cryoprotectant administration. And the type of viability assay used may affect the toxicity assessment.
A study of erythrocyte hemolysis by alkanols (alkanes having one −OH group), alkanediols (alkanes having two −OH groups), and glycerol (which has three −OH groups) showed that the degree of hemolysis was almost entirely dependent upon the shape-change induced in the erythrocytes, independent of the cryoprotectant used. The smaller the difference between the hydrophobicity of the solution and the hydrophobicity of the membrane, the greater was the extent of hemolysis and change in shape. Alkanols mainly affected membrane hydrophobicity, whereas alkanediols mainly affected solution hydrophobicity. Increasing chain length resulted in increasing hemolysis, whereas addition of a hydroxyl group to produce an alkanediol reduced hemolysis compared to the corresponding alkanol. Cryoprotectant concentrations that produced 100% hemolysis at 20ºC only produced 5-10% hemolysis at 4ºC. The authors suggested that the ability of formamide to reduce DMSO toxicity may be due to opposing effects of the two cryoprotectants on solution hydrophobicity [BIOCHEMICA ET BIOPHYSICA ACTA; Bakaltcheva,IB; 1280(1):73-80 (1996)]. The same mechanism may be behind the toxicity reduction for the combination of DMSO and ethylene glycol [REPRODUCTION, FERTILITY, AND DEVELOPMENT; Gautam,SK; 20(4):490-496 (2008)].
On the basis of the above erythrocyte hemolysis study it would seem that methanol would be the least toxic cryoprotectant. A study of fish oocytes showed that methanol concentrations above 6 Molar (but not below) resulted in protein damage or proteolysis [CRYOBIOLOGY; Lubzens,E; 53(3):398-399 {ABSTRACT 73} (2006)] Methanol is very hydrophobic and is a weak cryoprotectant, however.
Correlations have been found between cryoprotectant toxicity and destabilization of proteins in cell membranes [PHARMAZIE; Ivanov,IT; 56(10):808-809 (2001)]. DMSO perturbs cell membranes, possibly by displacement of water [BIOCHEMICA ET BIOPHYSICA ACTA; Westh,P; 1664(2):217-223 (2004)]. DMSO is more hydrophobic at higher temperature, so there is less DMSO membrane dehydration (toxicity) at lower temperature [BIOPHYSICAL JOURNAL; Sum,AK; 85(6):3636-3645 (2003)]. The membrane disruption by DMSO was slightly increased (rather than reduced) by addition of formamide for temperatures above 5ºC [BIOCHEMICA ET BIOPHYSICA ACTA; Anchordogny,TJ; 1104(1):117-122 (1992)]. DMSO oxidizes sulfhydryl groups, an effect which would be expected to decrease with lower temperature [BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS; Snow,JT; 64(1):441-447 (1975)]. DMSO, glycerol and especially propylene glycol can all form potentially toxic formaldehyde by non-enzymatic reactions [HUMAN REPRODUCTION; Karran,G; 11(12):2681-2686 (1996)]. DMSO, formamide and methanol have been shown to dissolve DNA at high concentrations and temperatures above 20ºC [BIOTECHNOLOGY AND BIOENGINEERING; Bonner,G; 68(3):339-344 (2000)].
Spectroscopic analysis of the enzyme lysozyme dissolved in concentrated solutions (close to solubility limits) of cryoprotectants showed complete loss of structure for DMF, DMSO and formamide, whereas some structure was preserved for the other cryoprotectants in the order glycerol > ethylene glycol > methanol [BIOTECHNOLOGY AND BIOENGINEERING; Knubovets,T; 63(2):242-248 (1999)]. The enzyme thermolysin is 95% inactivated by a 50% DiMethylFormamide (DMF) at 80ºC within 20 minutes, but a 50% DMSO solution only results in 10% inactivation under the same conditions. Addition of 20% glycerol reduces inactivation by DMF to 90% and trehalose reduces the inactivation by 80% [JOURNAL OF BIOTECHNOLOGY; Pazhang,M; 127(1):45-53 (2006)]. For mint shoot tips, sucrose reduces the toxicity of ethylene glycol and DMSO at 22ºC and of glycerol at 0ºC [CRYOBIOLOGY; Volk,GM; 52(2):305-308 (2006)]. Addition of 5% trehalose to 10% DMSO solution has been shown to enhance survival of nucleated cord blood cells from 7% to 25%, depending on the cell type [TRANSFUSION; Zhang,XB; 43(2):265-272 (2003)].
Cryoprotectant toxicity could be caused by denaturation of proteins (the protein denaturation hypothesis) [CRYOBIOLOGY; Arakawa,T; 27:401-415 (1990)], although there is evidence that proteins in general are not denatured at the temperatures and cryoprotectant concentrations relevent to vitrification [CRYOBIOLOGY; Fahy,G; 27:247-268 (1990)].
Recent discoveries provide clues as to why the substances giving the most powerful cryoprotection at low concentration are the most toxic. For kidney slices the cryoprotectants that vitrify most powerfully are those that hydrogen-bond most strongly to water, thereby interfering with the water-to-water hydrogen-bonding that is the basis of ice [CRYOBIOLOGY 48(1):22-35 (2004) & CRYOBIOLOGY 48(2):157-178 (2004)]. But those same cryoprotectants may also hydrogen-bond most strongly to proteins, causing the most unfolding and the most protein (enzyme) denaturation. The least toxic cryoprotectants prevent ice formation by weak hydrogen-bonding, but more importantly by colligative interference with ice formation. For this reason, a major breakthrough for organ cryopreservation was achieved by substituting ethylene glycol for propylene glycol in VS55 (also known as VS41A) solution (which is 3.1 Molar DMSO, 3.1 Molar formamide and 2.2 Molar propylene glycol in EuroCollins solution).
Although the greater toxicity of cryoprotectants that hydrogen-bond most strongly is explained by the protein denaturation hypothesis, it can also be explained by the dehydration damage hypothesis asserts that toxic cryoprotectants cause dehydration damage by binding to water molecules, thereby preventing the water molecules from properly hydrating proteins and other macromolecules. There is much evidence against this explanation. Dehydration damage is only caused by the removal of bound water. Bound water is 20 to 100 times more viscous than bulk water. Bound water can allow dehydrated cells with high protein content to vitrify without cryoprotectant. Powerful cryoprotectants toxic at low doses would find plenty of bulk water to hydrogen-bond. Any bound-water molecule hydrogen-bonded by a cryoprotectant molecule would be instantly replaced by a bulk water molecule.
Until recently, there was no means of predicting cryoprotectant toxicity. Producing cryoprotectant cocktails with low toxicity and high vitrification capability was a matter of random trial & error. However, with Patent 6,395,467 (inventors Dr. Gregory M. Fahy and Dr. Brian Wowk) a metric symbolized as qv* can be used to quantify toxicity as a function of molecular polar groups at concentration needed to vitrify. For
MW
moles of water
q = ------
=
-----------------------------------------------------------------
MPG
moles of polar groups on penetrating cryoprotectant
|
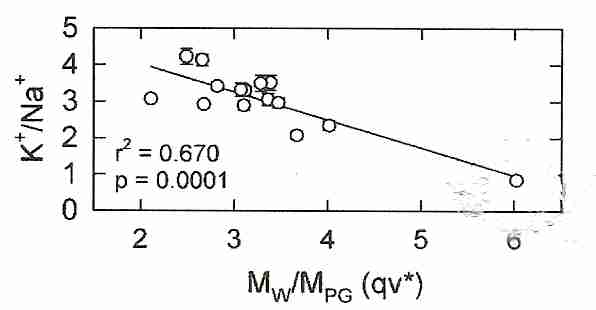
the quantity qv* can be used to control cryoprotectant toxicity — where qv* refers to q at a concentration needed to vitrify (Cv, the "v" in qv*) 5-10 ml of solution at a cooling rate of about 10ºC per minute (the "*" in qv*) down to glass transition temperature (Tg). Moles of polar groups (MPG) are arrived at by simple counting. For example, MPG of glycerol is 3 hydroxyl groups, MPG of ethylene glycol is 2 hydroxyl groups, and MPG of DMSO is 1 (the sulfinyl group of the sulfoxide). Toxicity and glass-forming ability varies linearly with qv*. For most cryoprotectants, qv* is in a range between 2 and 4, but DMSO has a qv* of about 6. (Note that v and * define the standard conditions of q — they are not multiplied times q.) Minimizing qv* results in vitrification with minimum toxicity.
Propylene glycol and formamide have two polar groups. The cryoprotectants with fewer polar groups will have a high q, and probably a high qv*. A solution with a high qv* will be vitrifying with fewer polar groups per water molecule, which probably means that there is stronger hydrogen bonding with water. Stronger hydrogen bonding means greater toxicity. For mixtures of cryoprotectants, qv* measures average hydrogen bonding strength for the mixture. It is suggested that the toxicity of a high qv* is due to a reduction in the water available for hydration of proteins, membranes and other cellular biomolecules.
Intuitively one might imagine that because the strongest vitrifying agents can achieve high vitrification at the lowest concentration — that the strongest vitrifying agents would be the least toxic agents (less water displaced and less total cryoprotectant in the tissues). But the qv* concept revealed that the penetrating cryoprotectants (ie, cryoprotectants that can cross cell membranes, such as propylene glycol and ethylene glycol) that require the highest concentrations to vitrify — and displace the most water — are actually the least toxic. Thus, ethylene glycol requires a higher concentration to vitrify than does propylene glycol, but is less toxic at the Cv, despite the fact that Cv for ethylene glycol is less than Cv for propylene glycol. The patent inventors speculated that the explanation for this effect is that although higher concentrations of weakly-hydrogen-bonding cryoprotectant result in lower concentrations of water, the lower concentrations of water can nonetheless more readily break bonds with cryoprotectant so as to hydrate life-critical molecules. Amides are helpful because they lower qv*, although they have a toxicity which is independent of qv* — a toxicity which is not additive to the toxicity of DMSO [CRYOBIOLOGY; 27;247-268 (1990) & CRYOBIOLOGY 48(1):22-35 (2004)].
(For example calculations of qv*, see the appendix.)
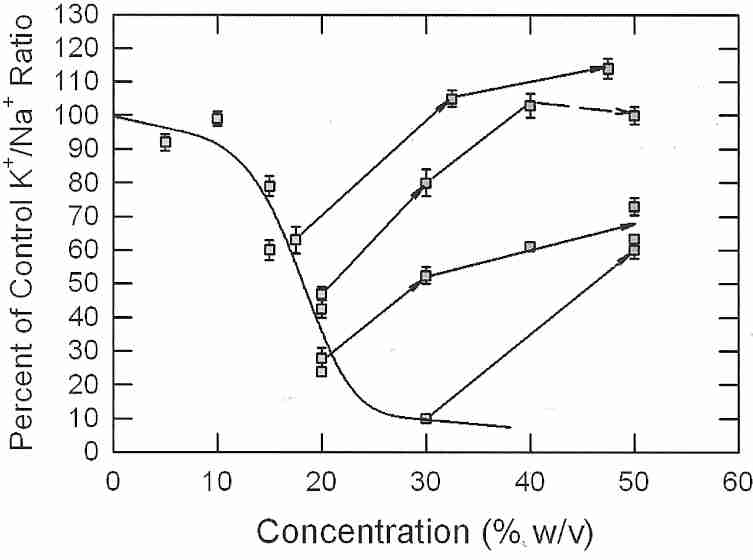
21st Century Medicine (21CM) researchers have had their best results from using combinations of cryoprotectants for good vitrification with least toxicity. Most CPAs, particularly amides, can reduce the toxicity of DMSO, when used in combination with DMSO. 21CM researcher Dr. GM Fahy's most effective and least toxic vitrification solution for nearly a decade was VS4 (Vitrification Solution 4), composed of 14% w/v DMSO, 14% w/v formamide, 11% w/v propylene glycol and 10% w/v colloid [TRANSPLANTATION 70(1):51-57 (2000)] — although this mixture can only vitrify at 1,000 atmospheres of pressure. Increasing propylene glycol to about 17% w/v allowed vitrification at 1 Atmosphere pressure, so the modified formula was called VS41A. When propylene glycol was replaced with a methoxylated compound, the resulting viability was slightly superior to that seen with the original vitrification solution (although this result was not statistically significant, and was not replicated). By replacing propylene glycol with ethylene glycol, however, the 21 CM researchers significantly increased viability — and the new cocktail is called Veg — ie, VS41A with ethylene glycol.
|
For nearly two decades it was assumed that formamide reduces the toxicity of DMSO, but this view was revised in 1990 with evidence that it is DMSO that reduces the toxicity of formamide [CRYOBIOLOGY; Fahy,GM; 27:247-268 (1990)]. Increasing concentrations of formamide added to renal cortex slices at 0ºC results in a curve of decreasing viability. But adding DMSO to 15% and 20% formamide raises renal slice viability to nearly 100%. At 0ºC DMSO is non-toxic up to concentrations of 30% for many cells and tissues, so it could be incorrect to think that not only is DMSO reducing the toxicity of formamide, but formamide is reducing the toxicity of DMSO.
Generally, combinations of cryoprotectants are less toxic than single-agent cryoprotectant solutions. For human articular chondrocytes, formamide is the most toxic single agent, but propylene glycol showed the least toxicity-reduction when used in combination with other cryoprotectant substances of any of the cryoprotectants tested [CRYOBIOLOGY; Almansoori,KA; 64(3):185-191 (2012)].
Concentration of penetrating cryoprotectant (and hence toxicity) can also be reduced by addition of nonpenetrating cryoprotectants such as large molecular-weight polymer (eg, polyvinylpyrrolidone or polyethylene glycol) or sucrose. Nonpenetrating cryoprotectants are too large to diffuse into cells, but they assist with vitrification of water (and inhibition of devitrification) in the extracellular space. Less cryoprotectant is needed inside cells than in the extracellular space because of dehydration (which drives water from cells into the extracellular space) and because cells naturally contain proteins that enhance vitrification. Patent 6,395,467 reports optimizing cryoprotectant vitrification while minimizing toxicity by a judicious use of (1) penetrating cryoprotectants (selection based on qv*) (2) non-penetrating cryoprotectants and (3) ice-blockers.
By adding ice-blockers and a judicious mixture of other CPAs to Veg the 21CM researchers developed a new vitrification solution called M22 (so-named because of the intention to introduce this vitrification cocktail to a biological specimen at Minus 22ºC.). M22 has been used to vitrify a rabbit kidney at −135ºC, which was subsequently rewarmed, transplanted into a rabbit and sustained the life of the rabbit as the sole functioning kidney [ORGANOGENESIS; Fahy,GM; 5(3):167-175 (2009)]. Ethylene glycol is a stronger glass-former than 1,3-propanediol [CRYOBIOLOGY; MacFarlane,DR; 27:345-358 (1990)], and by the qv* model would be expected to be less toxic (although it was not one of the CPAs tested in the published qv* studies) [CRYOBIOLOGY 48(1):22-35 (2004)]. Substituting 1,3-propanediol for ethylene glycol (or a mixture of the two) might bring further benefits along the lines of replacing propylene glycol with ethylene glycol, although 1,3-propanediol is a larger molecule which may not penetrate tissues as readily as ethylene glycol.
In general, cryoprotectant toxicities are lower at lower temperature, and may even become negligible if the cryoprotectants can be introduced at a low enough temperature. In perfusing the brain at the Cryonics Institute, ethylene glycol is introduced first, which can allow subzero temperature to be achieved (without freezing) before a DMSO/ethylene glycol mixture is introduced. The ethylene glycol also reduces the toxicity of DMSO.
One study found greatly reduced fertility of human oocytes from DMSO at 37ºC (human body temperature) for 30 minutes, but no reduction in fertility at 4ºC [HUMAN REPRODUCTION; Pickering,SJ; 6(1):142-143 (1991)]. A similar study of human neonatal dermal fibroblasts found 17% (v/v) DMSO caused a 50% loss of viability after 20 minutes at 37ºC, but at 4ºC 37% (v/v) DMSO was required to cause the same loss of viability in 20 minutes [CRYOBIOLOGY; Wang,X; 55(1):60-65 (2007)]. Unfortunately, cryoprotectants become increasingly viscous at low temperature, reducing their capacity to diffuse into tissues. So there is a trade-off of reduced toxicity and the requirement for greater exposure times to achieve tissue saturation at low temperature.
How important is cryoprotectant toxicity for cryonics? Can future technology easily replace denatured proteins? Enzymes should be easy to replace, and denatured membrane proteins may not cause so much structural damage as to prevent faithful reconstruction. Nonetheless, the goal of cryonics-directed cryobiological research should be to cause the least amount of damage possible, thereby lessening dependence upon future technology. Lessening damage lessens the chance of irreparable damage (ie, destruction).
(For more discussion of cryoprotectant properties, see
VITRIFICATION WITH NON-GLYCEROL CRYOPROTECTANTS.)
| Lecithin | Membrane |
|---|---|
![[Lecithin ]](lecithin.jpg)
|
![[Membrane]](Bilayer.jpg)
|
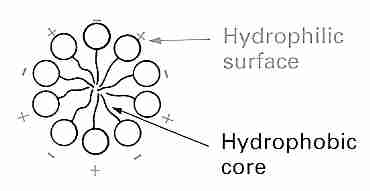
Membranes of cells & organelles (eg, mitochondria, nucleus, lysosomes, etc.) are primarily phospholipid bilayers containing proteins and cholesterol. The surrounding membrane of a cell (plasmalemma) contains, in addition, proteins with attached carbohydrates (glycoproteins) on the outer surface. A phospholipid has a hydrophilic ("water-loving", non-lipid, charged, polar) head (composed of a phosphate and a base) as well as a hydrophobic ("water-fearing", lipid, uncharged, nonpolar) tail of fatty acids. A glycerol "backbone" connects the head and the tail. The phospholipid lecithin (phosphatidylcholine), for example, connects glycerol (and the fatty acids attached to the glycerol) to a phosphate molecule (and the choline base attached to the phosphate). Stated another way, in a phospholipid a glycerol molecule attached to a phosphate group together serve as a means of connecting a hydrophilic base to two hydrophobic fatty acids. In a phospholipid bilayer hydrophilic head groups are oriented to the outside & inner surfaces where they can hydrogen-bond with water, whereas the hydrophobic tails are oriented to the inside of the membrane where they are attracted to other hydrophobic tails by van der Waals' force.
|
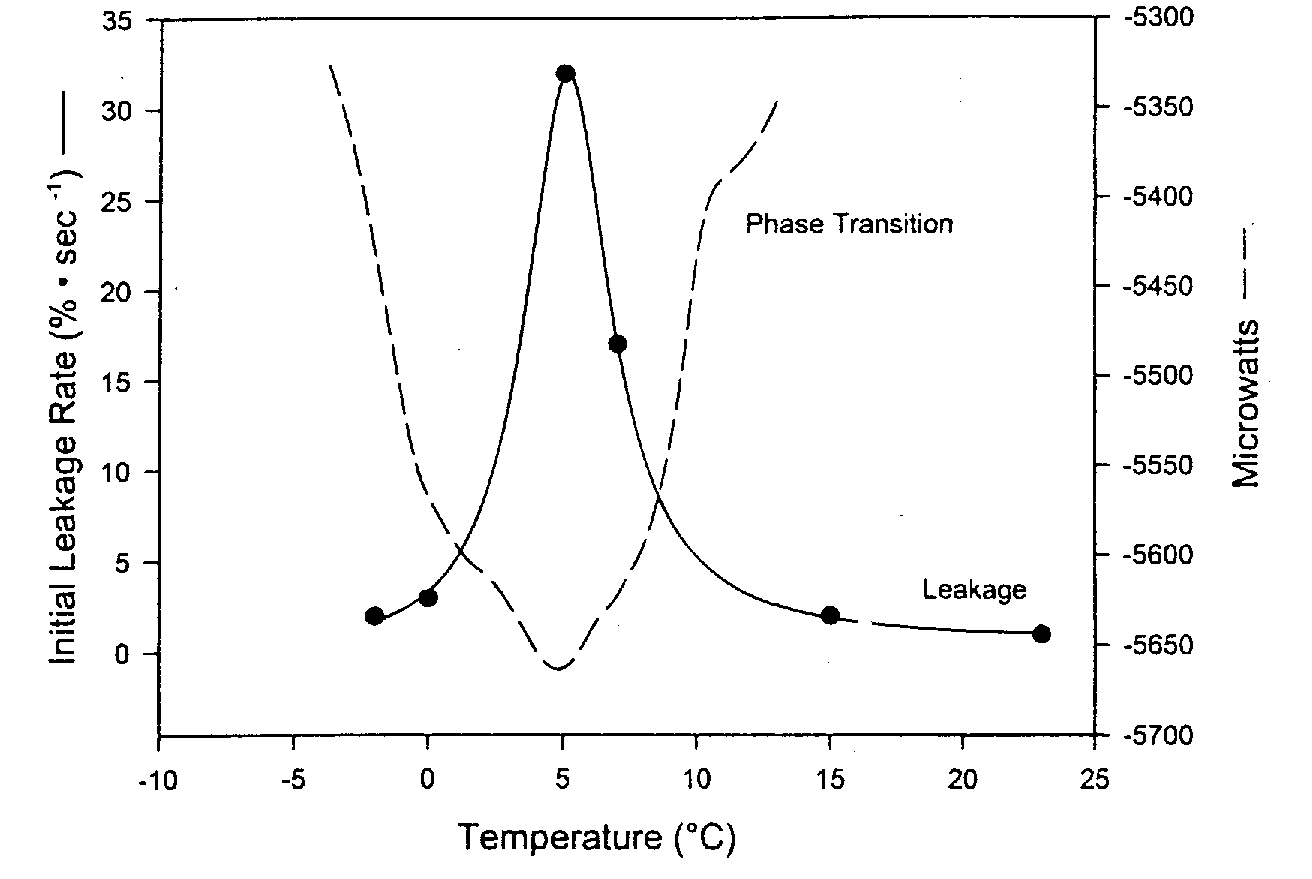
Phospholipid bilayer membranes have been called liquid crystals because the perpendicular organization of the phospholipids has a crystalline appearance. But the bilayer is described as a liquid because the lipid chains are loose and the phospholipids allow proteins in the membrane to move laterally or rotate about an axis perpendicular to the plane of the membrane. In contrast to this liquid phase, however, phospholipid bilayers can assume a more solid gel phase in which the lipid chains of the tail become more rigid, ordered and tightly packed. This phase transition (called the thermotropic phase transition) is much like the solidification of grease in a frying pan. Analogous to the phase transition in which solid ice melts to form liquid water, the thermotropic phase transition temperature is referred to as a melting temperature, Tm (a range of temperatures, between 0ºC and 10ºC in the diagram example).
Upon lowering or elevating temperature, maximum damage and leakage of membranes is seen at the phase transition temperature due to packing defects in the phase boundaries between the liquid and gel portions of the membrane. Far less leakage is seen below (and above) the thermotropic phase transition temperature. Surfactants (detergents) can worsen this damage. More rapid cooling and warming through Tm reduces membrane damage and leakage [CRYOBIOLOGY; Hayes,LM; 42(2):88-102 (2001)]. Higher phase transition temperatures (Tm) are seen for membranes containing higher percentages of saturated fat. Some organisms can actually increase the unsaturation of the lipids in their membranes in response to lowering temperature, thereby lowering Tm and protecting against the membrane damage that occurs at Tm.
Dehydration and dehydration injury is relevant to all forms of cryoprotection. In vitrification where water is replaced by cryoprotectant there will at least be temporary dehydration associated with the "shrink-swell cycle" due to the fact that water leaves cells faster than cryoprotectants can enter. A phosphatidylcholine polar head-group typically hydrogen-bonds with about ten water molecules. Hydration of head groups significantly affects Tm. If water is removed Tm increases considerably because the head groups pack more tightly together.
|
Liposomes are spheres of phospholipids which are used as experimental models for understanding membrane properties. Tm for hydrated liposomes of phosphatidylcholine is about −10ºC, but dehydration of the liposomes increases Tm to about 60ºC. Addition of sugars such as trehalose considerably reduces Tm for dehydrated liposomes (and phospholipid bilayers) by hydrogen-bonding between the head groups and thereby allowing for freer movement of the hydrophobic tails [BIOPHYSICAL JOURNAL; Koster,KL; 78(4):1932-1946 (2000)]. Lowering Tm with sugar can mean that dehydration will not elevate Tm which would cause Tm to pass through ambient temperature, resulting in membrane damage. Trehalose can lower Tm enough to prevent membrane damage during lyophilization (freeze-drying).
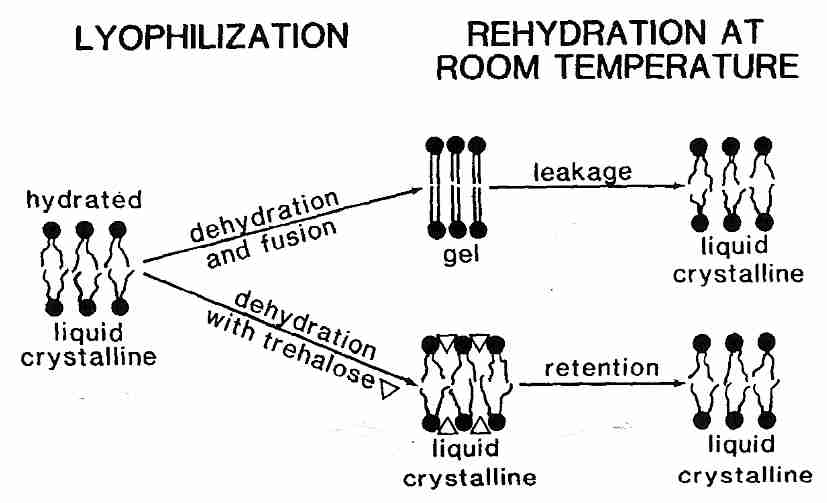
|
Reduction of Tm, however, is not sufficient to prevent dehydration injury to liposomes & phospholipid bilayers. With or without sugars, dehydration of liposomes causes them to pack tightly together and fuse. Fusion means that many small lysosomes coalesce to form a few large lysosomes. Fusion of cell membranes is clearly damaging to tissues and causes considerable leakage of cell contents. Fusion can be prevented, however, by vitrification.
Pure sucrose melts & decomposes to carmel above 186ºC, but as a sugary liquid can be supercooled to solidify to a (non-crystalline) glassy state at 65ºC. This phase transition temperature is called Tg, the glass transition temperature. Mixtures of water & sucrose can also be syrupy liquids above Tg and glassy solids below Tg. With increasing amounts of water the Tm of the sucrose-water mixture drops to well below 0ºC with as little as 10% water [BIOPHYSICAL JOURNAL; Sun,WQ; 70(4):1769-1776 (1996)]. Dehydration of liposomes in a sugar medium elevates Tg. Devitrification due to a rising Tg which passes through ambient temperature in association with dehydration is even more damaging than a rise of Tm above ambient temperature. The best strategy is to keep Tm well below ambient temperature and Tg well above ambient temperature. Pure trehalose has a Tg of 120ºC, which is well above that of sucrose (75ºC) [THE JOURNAL OF PHYSICAL CHEMISTRY B; Lerbret,A; 109(21):11046-11057 (2005)] or glucose (30ºC). Thus, although trehalose is not better than sucrose in its ability to depress dehydration Tm, the high Tg of trehalose (along with its resistance to hydrolysis) makes it the preferred sugar for use in dehydration preservation.
Vitrified sugars interact with membrane lipids in a different manner than do non-vitrified sugars. Specific interactions (eg, hydrogen bonding) between the sugars and phospholipids are less important for vitrified sugars, which means that membranes in a vitrified sugar behave much the same whether the sugar is trehalose, sucrose, glucose or sorbitol. Experiments with POPC (1-Palmitoyl-2-OleoylPhosphatidyl Choline, a mixed-chain, monosaturated phosphatidylcholine) showed that dehydration of POPC in the absence of sugars elevated Tm from −3ºC to 61ºC. But in the presence of any of the combination of sugars tested Tm never rose above 6ºC regardless of the amount of dehydration. Tm in the presence of vitrified sugars was lowered to approximately −25ºC, [BIOCHEMICA ET BIOPHYSICA ACTA; Koster,KL; 1193(1):143-150 (1994)].
(For more information on trehalose, see cryopreservation with sugars.)
Chilling injury and cold shock reduce viability at low temperatures above those that induce freezing. In general, chilling injury refers to damage induced in cells held at critical temperatures below temperatures at which cells normally function, whereas cold shock refers to reduced viability due to either a rapid decrease in temperature or a large decrease in temperature. There is some overlap in the effect on cellular organelles of cold shock and chilling injury, especially in cell membranes. There is also some confusion in the terminology used, at least partially associated with the lack of clarity concerning the mechanisms. Chilling injury and cold shock are probably greater obstacles to cryopreserving fish embryos than vitrification or cryoprotectant toxicity.
Heat shock leads to a well-defined unfolding or denaturing of proteins — heat shock induces heat-shock proteins (chaperones) to maintain protein folding. Cold shock has a wider range of effects that are less well defined. Cold shock has been studied most thoroughly using Bacillus subtilis bacteria. Cold shock most immediately impacts membrane-bound lipids, protein conformation, and nucleic acid conformation. mRNA translation is inhibited. Cold-shock proteins are induced, and there is increased synthesis of more unsaturated fatty acids to increase membrane fluidity [PHILOSOPHICAL TRANSACTIONS OF THE ROYAL SOCIETY B; Weber,MWH; 357:895-907 (2002)]. Initial mRNA translation appears to be the key control point for the cold-shock response in mammalian cells. Membrane-bound enzyme activity is inhibited, and diffusion rates are reduced. Cold shock proteins (very different from those in bacteria) may recruit mRNA and ribosomes to the cytoskeleton for translation [THE BIOCHEMICAL JOURNAL; Al-Fageeh,MB; 397(Pt 2):247-259 (2006)].
|
Chilling injury increases with exposure time at critical temperatures and, in fact, rapid cooling through the critical temperature range can be a means to reduce chilling injury [CRYOBIOLOGY; Mazur,P; 29(1):39-68 (1992)]. In his breakthrough vitrification of 50,000 cell Drosophila embryos (embryos already differentiated into tissues & organs, including muscle & nerve) with ethylene glycol, Peter Mazur found that the embryos were extremely sensitive to chilling injury and became increasingly so as temperature falls to −25ºC. A critical component to successful Drosophila embryo vitrification was out-racing chilling injury with cooling rates of at least 20,000ºC per minute [SCIENCE; Mazur,P; 258:1932-1935 (1992)]. Fish embryos which are vulnerable to both chilling sensitivity and cold shock, cannot be cryopreserved by such rapid cooling [THERIOGENOLOGY; Liu,X; 55(3):1719-1731 (2001)].
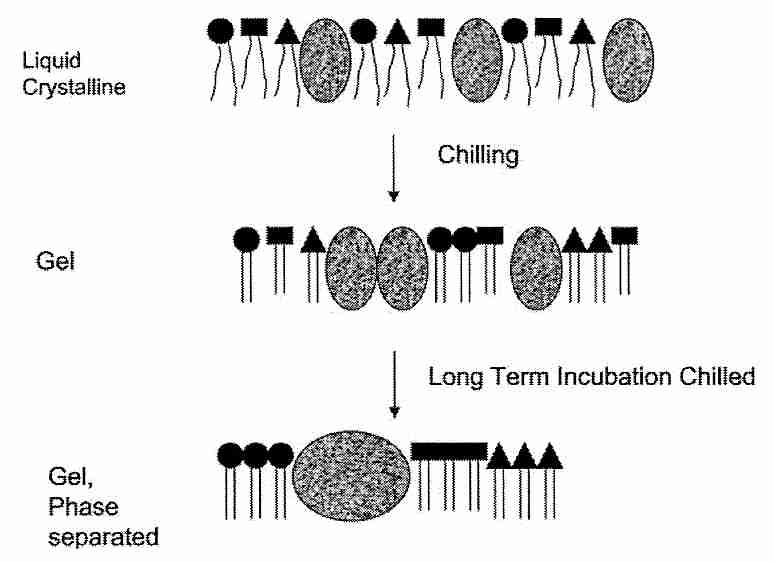
One mechanism of chilling injury in animal cells is probably due to phase transitions in cell membranes [CRYOBIOLOGY 42(2):88-102 (2001)]. Lipids in cell membranes would be expected to undergo a liquid-to-gel phase transition in a range between 0ºC and 20ºC, the temperature range of maximum chilling injury. Chilling sensitivity has been reduced in plants [NATURE 356:710-713 (1992)] and in sheep oocytes [MOLECULAR REPRODUCTION AND DEVELOPMENT; Zeron,Y; 61(2):271-278 (2002)].
Most of the membrane damage associated with chilling injury, however, is not believed to be due to the leakiness associated with a single Tm (thermotropic phase transition temperature — see previous section), however. Most of the chilling injury is attributed to lateral segregation of proteins & phospholipids within membranes. Cell membrane phosopholipids are heterogenous, being composed of different bases in the head groups and different fatty acids in the tails. More important, however, is the existence of detergent-insoluble rafts ("moving platforms") in cell membranes where there are high concentrations of sphingolipids & cholesterol along with specialized proteins [NATURE; Simons,K; 387:569-572 (1997)]. Sphingolipids (the simplest of which are the ceramides) are composed of a sphingoid base having highly saturated fatty acid chains. A ceramide can be released into the cell by enzymes, and act as a signalling molecule within the cell. Ceramides have a much higher Tm than do phospholipids, which contributes to the heterogenous dysfunction of the cell membrane upon cooling. Lateral phase separation and aggregation of lipid and protein components during chilling considerably disrupts membrane organization.
Platelets are exceptionally vulnerable to chilling injury and have served as models for cold-induced dysfunction [BIOCHEMICA ET BIOPHYSICA ACTA; Gosset,K; 1660(1-2):7-15 (2004)]. Much as the sugar trehalose can reduce dehydration injury, it can protect membranes against the chilling injury of graft aggregation by hydrogen bonding with phospholipid head groups and proteins [CHEMISTRY AND PHYSICS OF LIPIDS; Crowe,JH; 122(1-2):41-52 (2003)]. For this reason one way of avoiding chilling injury in cryopreservation has been to cool rapidly so as to minimize time spent in the phase transition region. Chilling injury has been reduced in fish & pig embryos by removing lipids (yolk) [CRYOBIOLOGY 39(3):236-242 (1999)]. The sugar trehalose reduces membrane component aggregation possibly by binding "microdomains" together and thereby limiting their mobility. At the phospholipid bilayer of cell membranes trehalose is able to displace water molecules bound to carbonyls [BIOPHYSICAL JOURNAL; Amalfa,F; 78(5):2452-2458 (2000)]. Mammalian cells have been protected from damage by freezing and dehydration with trehalose by creating pores in the cells to allow for trehalose loading [NATURE BIOTECHNOLOGY; Eroglu,A; 18(2):163-167 (2000)], by cloning a facilitated trehalose transporter gene from an insect [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Kikawada,T; 104(28):11585-11590 (2007)] or by transfecting the cells with genes that gives the cells the capability of trehalose synthesis [NATURE BIOTECHNOLOGY; Guo,N; 18(2):168-171 (2000)].
Along the same lines, membrane leakiness has been exploited by using DMSO to facilitate entry of trehalose into cells for protection of intracellular membrane surfaces [DIABETES 46:519-523 (1997)]. Toxicity of DMSO upon rewarming should not be of so much concern in cryonics applications. In fact, membrane damage by chilling injury may affect viability much more than general structure and therefore be of less concern in cryonics.
Antifreeze glycoprotein from polar fish reduces chilling injury in platelets by inhibiting aggregations in the membrane, but the technique cannot be used clinically because of the difficulty of removing the antifreeze proteins [CRYOBIOLOGY 43(2):114-123 (2001)] — a consideration of less concern to cryonicists who are more interested in preservation of structure than in viability on rewarming. Methanol protects zebrafish embryos from chilling injury, a benefit speculated to be due to possible depression of phase transition temperatures in the lipid membranes [THERIOGENOLOGY; Zhang,T; 59(7):1545-1556 (2003)].
Another cause of chilling injury (or cold shock) is protein denaturation. Just as proteins are denatured by heat, proteins can also be denatured by cooling. The exposure of hydrophobic regions of proteins becomes less energetically unfavorable at lower temperatures and thus proteins will unfold as temperatures are lowered. Some of the denatured proteins are antioxidant enzymes, especially forms of SuperOxide Dismutase (SOD), which may explain why better survival for lyophilization (freeze-drying) is obtained in the absence of air. Again, rapid cooling can be an effective means of reducing protein denaturation. Both heat shock proteins and trehalose can protect proteins and help reduce unfolding. Again, if the injury is primarily by denaturing enzymes, this effect on viability may be of much less concern to cryonicists insofar as structural damage would be minimal.
Although chilling sensitivity has been reduced in plants by increasing the degree of fatty acid unsaturation [NATURE 356:710-713 (1992)], much of the chilling injury (or cold shock) in plants has been attributed to free radical damage [JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 47:2410-2414 (1999)]. Evidence for free radical damage during chilling has also been seen in houseflies [CRYOBIOLOGY 33(4):447-458 (1996)] and sperm (sperm membranes have a high polyunsaturated fatty acid content) [MOLECULAR REPRODUCTION AND DEVELOPMENT; Neild,DM; 72(2):230-238 (2005)].
Uncoupling of ATP synthesis from electron transport chain mitochondrial enzymes by chilling (or cold shock) has been demonstrated — and coincident with the increased oxidative stress is a reduction of antioxidant enzyme activity due to chilling. The antioxidant enzyme catalase shows a marked decline in activity with lowering of temperature, whereas peroxidase activity remains unchanged — resulting in hydrogen peroxide accumulation. Chill-tolerant plants show increased levels of catalase production with lowering temperature. Increased calcium influx can activate phospholipases that break-down membranes by releasing fatty acids. Exclusion of air (oxygen) has been shown to reduce chilling injury in plants and insects. Free radicals can destroy structure if they are permitted to operate for extended periods. It seems likely that chilling injury due to free radicals must also occur to some extent in mammalian tissues due to cold ischemia as well as similar mechanisms to those seen in plants. Oxygen is more soluble in water at low temperature, which contributes to low temperature damage by reactive oxygen species. Trehalose protects cells and proteins from oxidative damage, whereas sucrose does not [JOURNAL OF BIOLOGICAL CHEMISTRY; Benaroudj,N; 276(26):24261-24267 (2001)].
Microtubule polymerization in oocytes is very temperature sensitive, and complete microtubule depolymerization can occur just above 0ºC [BIOLOGY OF REPRODUCTION; Aman,RR; 50(1):103-110 (1994)]. In some cases, repolymerization of the meiotic spindle occurs on rewarming [FERTILITY AND STERILITY; Ciotti,PM; 91(6):2399-2407 (2009)], but in other cases irregular chromosomal configurations and abnormal tubulin organization remains after rewarming [FERTILITY AND STERILITY; Songsasen,N; 77(4):818-825 (2002)].
Cooling experiments of rabbit kidney cortical slices in vitrification solution have been conducted in which viability (K+/Na+ ratios) was used as an index of chilling injury (or cold shock) have indicated a linear increase in chilling injury from 0ºC to −85ºC. It seems unlikely that lipid phase transition would be responsible for this chilling injury. Hypertonic solutions in the range of 1.2 to 1.5 times isotonicity completely abolished chilling injury between 0ºC to −22ºC. Chilling injury down to −135ºC was minimized (85-90% viability) by cooling to −22ºC at 1.2X hypertonicity and further cooling to −135ºC with 1.5X hypertonicity [CRYOBIOLOGY; Fahy,GM; 48(2):157-178 (2004)]. Shrinking cells somewhat evidently protects them against chilling injury.
Cryonics research has been using intracellular K+/Na+ ratios (potassium-sodium ratios) to assess the effectiveness of vitrification formulas. If intracellular K+/Na+ ratios are normal after application of cryoprotectant and/or cooling followed by rewarming, that would be an indication that cell membranes have not been breached by ice formation. Damaged membranes would give intracellular K+/Na+ ratios matching those of the extracellular fluid. But intracellular K+/Na+ ratios should also be an index of viability, because if cryoprotectant has poisoned a cell and killed it, there can be no ATP production or functionality of the sodium pump.
When tissue samples are exposed to cryoprotectants and low temperatures, sodium pumping is incapacitated and the intracellular K+/Na+ ratios approach extracellular levels as a result of ion leak. If vitrification has been effective, when the tissues are re-warmed and the cryoprotectant is washed-out, the sodium pump will restore the normal K+/Na+ ratio. To allow this process to occur, tissue slices are incubated in warm oxygenated growth media. Within 40-60 minutes the tissue slices will achieve K+/Na+ ratios in proportion to their viability (by definition). Thus, if the tissue recovers 85% of the normal intracellular K+/Na+ ratio it is said to be 85% viable. This index of viability measures both sodium pump functionality and mitochondrial ATP-generating capacity, without distinguishing which is being affected. Poisoning the tissue sodium pumps with ouabain will result in tissue intracellular K+/Na+ ratios matching that of the environment within about 30 minutes.
The biggest advantage of K+/Na+ assay is that it is inexpensive and easier to measure than structural damage seen in micrographs. The assays are quantitative and can be obtained quickly. It is certainly less costly than taking Electron Micrographs (EMs), although the latter is the ultimate standard for the absence of structural damage due to ice. Both K+/Na+ ratios and EMs — viability and structural integrity measures — require rewarming (and possible devitrification). If it were economically feasible to assess histological structure at −130ºC — cryohistology not requiring rewarming — that would give the best assessment of the quality of structural preservation in long-term storage. Nonetheless, absence of structural damage is certainly affirmed if samples are rewarmed without vitrification for histological preparations that show no ice damage.
Another tool for assessing vitrification (lack of ice formation and hence lack of structural damage due to ice) is Differential Scanning Calorimeter (DSC), which can detect "thermal signatures" of ice formation and glass transition in small tissue samples (1 mg to 10 mg). Cryobiologist Pierre Bouton used 0.5% ice formation as the lower limit of structural damage, but 21st Century Medicine (21CM) uses the more stringent figure of 0.2%. (In fact, there is no definite proof for either figure, although certainly zero percent ice formation would result in zero damage due to ice.) Just as small samples can be taken and rewarmed for EMs, small samples can be taken and rewarmed for DSC analysis. Although DSC has the advantage of being quantitative, it is still a less definitive indicator of absence of structural damage than EMs as long as ice formation is greater than zero.
The fact of injury or loss of viability is often not helpful in determining the source of that injury. Injury can occur during cryoprotectant loading, during cooling, during rewarming or during cryoprotectant unloading. Chilling injury, osmotic injury, cryoprotectant toxicity and devitrification all reduce viability. Viability may be lost by neurons, but not glial cells, thereby masking loss of viability of neurons, although electron micrographs are useful for determining if distinct cell types are being damaged. Knowing the source of injury is of critical importance in seeking a remedy and it is often the case that neither K+/Na+ assays nor electron micrographs are helpful in this regard. DSC, however, can be useful in identifying Tm values and associated chilling injury [CRYOBIOLOGY; Hayes,LM; 42(2):88-102 (2001)].
In my opinion cryonics research should use K+/Na+ assays as the first test of cryoprotective efficiency and cryonics protocol. K+/Na+ ratios which are 40% of normal will indicate intact membranes (lack of structural damage) even if viability is low. Then emphasis should be placed on DSC (where feasible), Light Micrographs (LMs) and ultimately EMs. Only after perfect EMs are attained can we be assured that structural damage has been eliminated. At that time we would have the luxury of returning the emphasis to K+/Na+ ratios directed at obtaining K+/Na+ ratios approaching 100% of normal (full viability).
Table 1 of Cryobiology 21:407 (1984) gives the concentration needed to vitrify for 1,3-propanediol as 57%w/v, so I am assuming we have 57 grams of 1,3-propanediol in the RPS-2 (Renal Perfusion Solution two) carrier solution. * indicates multiplication and / indicates division.
The density of 1,3-propanediol is 1.0597 gm/mL therefore volume of 1,3-propanediol in 100 mL of solution is
57 * (1/1.0597) = 53.79 milliliters (mL)
plus 2.00 mL of RPS-2 components [from Table 2 of Cryobiology 48:22 (2004)] gives 55.79 mL
Therefore the volume of water is
100 mL − 55.79 mL = 44.21 mL
Water density is 1gm/mL and the molar mass of water is 18.015 gm/mole
Therefore 44.21 grams of water is
44.21 gm * (1/18.015 gm/mole) = 2.454 moles water
The molar mass of 1,3-propanediol is 76.09 therefore we have
57 gm * (1/76.09) = 0.7491 moles 1,3-propanediol
There are two polar groups per molecule of 1,3-propanediol
Therefore, per 100 mL of solution we have:
qv* = Mw/Mpg = 2.454/(2*0.7491) = 1.64
which is close to the value of 1.6 shown in Table 1 of patent 6,395,467
This is a calculation of qv* for solution 14 from Table 1 of Cryobiology 48:22 (2004). Solution 14 is equal parts ethylene glycol (EG) and propylene glycol (PG) in a 6% solution of PolyVinylPyrrolidone (PVP) in RPS-2 (Renal Perfusion Solution two) carrier solution.
(In this calculation, I allow decimal places to accumulate and I do rounding at the end. * indicates multiplication and / indicates division. )
The value of the concentration needed to vitrify for solution 14 is shown as 46%w/v in Table 1 of Cryobiology 48:22 (2004).
For solution 14 there are 20 grams EG, 20 grams of PG and 6 grams PVP in RPS-2 per 100 milliliters (mL) of solution
The density of EG is 1.1132 gm/mL therefore the volume is
20 mL * (1/1.1132 gm/mL) = 17.9662 mL
The density of PG is 1.036 gm/mL therefore the volume is
20 mL * (1/1.1036 gm/mL) = 19.305 mL
Density of PVP is 1.28 gm/mL or 0.782 mL/gm as per Table 2 therefore volume is
6 mL * 0.782 mL/gm = 4.692 mL
Solid components of RPS-2 from Table 2 is 2.00 mL in volume
Therefore the total volume of EG+PG+PVP+RPS-2 is
17.9662 mL + 19.305 mL + 4.696 mL + 2.00 mL = 43.9672 mL
Therefore the volume of water is
100 mL − 43.9672 mL = 56.0328 mL
This involves the simplifying assumption that if you add 17.9662 mL EG to 19.305 mL PG, 4.696 mL of PVP and RPS-2 you get 100 mL of solution, which in practice does not tend to happen, although it is roughly accurate.
Water density is 1gm/mL and molar mass of water is 18.015 gm/mole
Therefore 56.0328 grams of water is
56.0328 gm * (1/18.015 gm/mole) = 3.11034 moles water
The molar mass of EG, PG and PVP are 62.068, 79.06 and 40,000 respectively, therefore we have
20 gm * (1/62.068 gm/mole) = 0.332956 moles EG
20 gm * (1/79.06 gm/mole) = 0.252972 moles PG
6 gm * (1/40,000 gm/mole) = 0.00015 moles PVP
There are two polar groups per molecule of EG and PG, but I have not got a clue how many polar groups there are per molecule of PVP. If it was 1 polar group on PVP (highly unlikely) the effect of PVP would be negligible. Insofar as I have no value for PVP polar groups, I will ignore it and assume that the number of moles of polar groups is equal to the number of moles of EG polar groups. PVP is too large of a molecule to penetrate cell membranes, so I am assuming that only the penetrating cryoprotectants are relevant.
(0.332956 moles EG + 0.252972 moles PG) * 2 = 1.17186 moles polar groups
Therefore, per deciliter of solution I have:
qv* = (moles water)/(moles polar groups) = Mw/Mpg = 3.11034/1.17186 = 2.6542
which is close to the value of 2.67 shown in Table 1 of Cryobiology 48:22 (2004).